A Novel Plasmid-Based Co-Tethered Transcription Platform for High Yield, High Purity mRNA Synthesis
Purnima Mala, Ruptanu Banerjee, Amin Abek, James Forster III, Aniruddha Pinjari, Ashish A. Kulkarni, and Craig T. Martin, Nucleic Acids Res, in press, 2025.
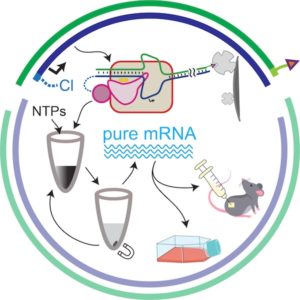
This work aims to improve RNA synthesis and manufacturing, exemplified by T7 RNA polymerase-driven in vitrotranscription. We developed a novel plasmid-compatible co-tethering strategy that functionally couples RNA polymerase to its promoter DNA immobilized on a solid matrix. As demonstrated recently, co-tethering enhances promoter binding, increases RNA yield, and suppresses RNA re-binding, especially under high-salt conditions, thereby reducing dsRNA by-products. The system leverages asymmetric end-labeling of linearized plasmid DNA using a simple “Klenow fill-in” reaction with modified nucleotides, enabling stable attachment of DNA to both RNA polymerase and solid support (magnetic beads). The immobilized co-tethered polymerase-DNA complex supports efficient transcription initiation in high-salt environments (which further reduces RNA re-binding), yielding RNA of high purity. Co-tethered complex remains functionally stable over extended storage and multiple transcription cycles (10-20 rounds), re-using the enzyme-DNA catalyst. Transcripts of lengths (0.8, 5.6, and 8.6 kb) are efficiently produced. Highly sensitive in vitro assays with immune cells confirm low immunogenicity and strong translational output, while in vivo validation using a novel Matrigel-plugged mouse model demonstrates robust expression and safety. With a simple modification to the DNA template, the reusable, co-tethered enzyme–DNA catalytic complex streamlines mRNA manufacturing by producing RNA of higher purity from the outset.
PMID: xxxxx PMCID: xxxxx DOI:https://doi.org/10.1093/nar/gkaf1355