High-salt transcription from enzymatically gapped promoters nets higher yields and purity of transcribed RNAs
Kithmie MalagodaPathiranage, Elvan Cavac, Tien-Hao Chen, Bijoyita Roy, Craig T Martin
Nucleic Acids Research, 51(6), e36 (12 pages)
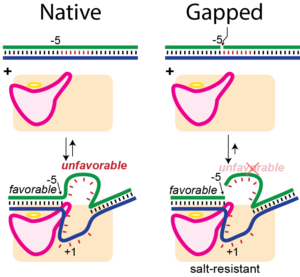
T7 RNA polymerase is commonly used to synthesize large quantities of RNA for a wide variety of applications, from basic science to mRNA therapeutics. This in vitro system, while showing high fidelity in many ways, is also well known for producing longer than encoded RNA products, particularly under high-yield reaction conditions. Specifically, the resulting product pool is contaminated by an often disperse collection of longer cis-primed extension products. In addition to reducing yield via the conversion of correctly encoded RNA to longer products, self-primed extension generates partially double-stranded RNAs that can trigger the innate immune response. Extensive and low-yield purifications are then required to produce therapeutic RNA. Under high-yield conditions, accumulating concentrations of RNA effectively compete with promoter DNA for polymerase binding, driving self-primed extension at the expense of correct initiation. In the current work, we introduce a simple and novel modification in the DNA to strengthen promoter binding, shifting the balance back toward promoter-driven synthesis and so dramatically reducing self-primed extension. The result is higher yield of the encoded RNA at the outset and reduced need for extensive purifications. The approach can readily be applied to the synthesis of mRNA-length products under high-yield conditions.
PMID: xxxxx. DOI: https://doi.org/10.1093/nar/gkad027