High salt transcription of DNA co-tethered with T7 RNA polymerase to beads generates increased yields of highly pure RNA
Elvan Cavac, Luis E. Ramírez-Tapia, and Craig T. Martin, J Biol Chem, 297(3), 100999, 2021. doi: 10.1016/j.jbc.2021.100999
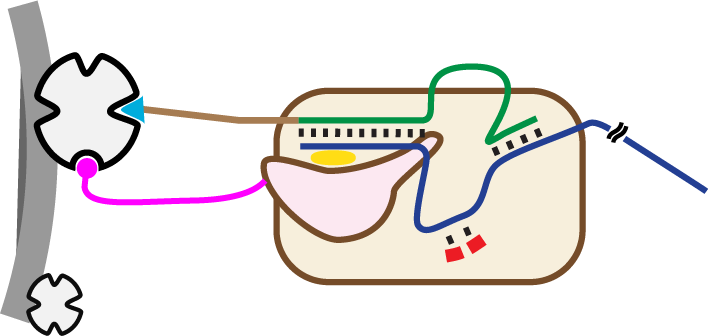
High yields of RNA are routinely prepared following the two-step approach of high-yield in vitro transcription using T7 RNA polymerase followed by extensive purification using gel separation or chromatographic methods. We recently demonstrated that in high-yield transcription reactions, as RNA accumulates in solution, T7 RNA polymerase rebinds and extends the encoded RNA (using the RNA as a template), resulting in a product pool contaminated with longer-than-desired, (partially) double-stranded impurities. Current purification methods often fail to fully eliminate these impurities which, if present in therapeutics, can stimulate the innate immune response with potentially fatal consequences. In this work, we introduce a novel in vitro transcription method that generates high yields of encoded RNA without double-stranded impurities, reducing the need for further purification. Transcription is carried out at high salt conditions to eliminate RNA product rebinding, while promoter DNA and T7 RNA polymerase are co-tethered in close proximity on magnetic beads to drive promoter binding and transcription initiation, resulting in an increase in overall yield and purity of only the encoded RNA. A more complete elimination of double-stranded RNA during synthesis will not only reduce overall production costs, but also should ultimately enable therapies and technologies that are currently being hampered by those impurities.
PMID: 34303704 PMCID: PMC8368030 DOI: 10.1016/j.jbc.2021.100999