Structural Confirmation of a Bent and Open Model for the Initiation Complex of T7 RNA Polymerase
Rosemary S. Turingan, Cuihua Liu, Mary E. Hawkins, & Craig T. Martin, Biochemistry 46, 1714-1723, 2007.
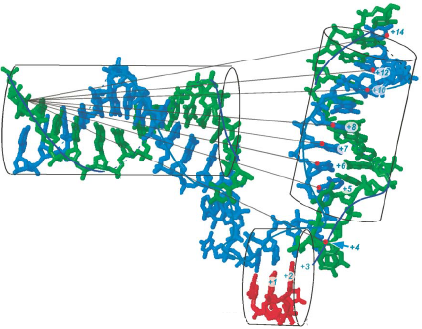
T7 RNA polymerase is known to induce bending of its promoter DNA upon binding, as evidenced by gel-shift assays and by recent end-to-end fluorescence energy transfer distance measurements. Crystal structures of promoter-bound and initially transcribing complexes, however, lack downstream DNA, providing no information on the overall path of the DNA through the protein. Crystal structures of the elongation complex do include downstream DNA and provide valuable guidance in the design of models for the complete melted bubble structure at initiation. In the current study, we test a specific structural model for the initiation complex, obtained by alignment of the C-terminal regions of the protein structures from both initiation and elongation and then simple transferal of the downstream DNA from the elongation complex onto the initiation complex. FRET measurement of distances from a point upstream on the promoter DNA to various points along the downstream helix reproduce the expected helical periodicity in the distances and support the model's orientation and phasing of the downstream DNA. The model also makes predictions about the extent of melting downstream of the active site. By monitoring fluorescent base analogs incorporated at various positions in the DNA we have mapped the downstream edge of the bubble, confirming the model. The initially melted bubble, in the absence of substrate, encompasses 7-8 bases and is sufficient to allow synthesis of a 3 base transcript before further melting is required. The results demonstrate that despite massive changes in the N-terminal portion of the protein and in the DNA upstream of the active site, the DNA downstream of the active site is virtually identical in both initiation and elongation complexes.
PMID: 17253774 PMCID: PMC2517905 DOI: 10.1021/bi061905d