Topological and conformational analysis of the initiation and elongation complex of T7 RNA polymerase suggests a new twist
Karsten Theis, Peng Gong and Craig T. Martin, Biochemistry, 43, 12709-12715, 2004
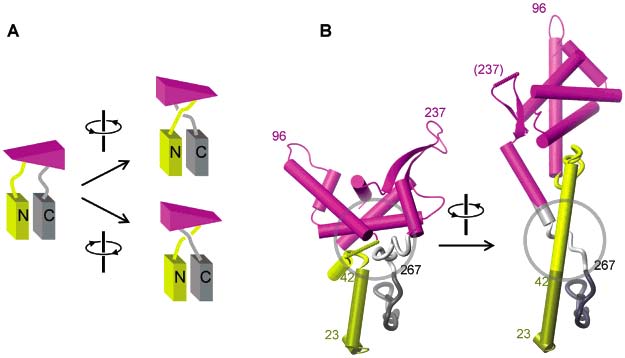
The N-terminal domain of T7 RNA polymerase undergoes large conformational changes in the transition from transcription initiation to elongation. The rigid body displacement of parts of the N-terminal domain (residues 72-152 and 204-258) has been described as a screw motion composed of a rotation by 140¡ and a translation of >20 along the rotation axis. Protein-protein interactions between residues 23-42 and the C-terminal domain are present in both the initiation and the elongation complex. Assuming that these interactions are retained during the transition between the two states, we find that topological constraints require a right-handed 220¡ screw motion of the N-terminal rigid body rather than the proposed 140¡ left-handed screw motion. In the initiation complex, a loop (residues 153-203) extruding from the N-terminal rigid domain wraps around the N-terminal 30 residues. Assuming the Nterminal rigid domain stays folded during the transition, the N-terminus has to pass through this loop before the rigid domain can undergo the translation leading to the elongation complex. Based on these topological constraints, we suggest an alternate sequence of conformational changes leading from transcription initiation to elongation in T7 polymerase.
PMID: 15461442 DOI: 10.1021/bi0486987