Pre-steady State Kinetics of Initiation of Transcription by T7 RNA Polymerase - A New Kinetic Model
Iaroslav Kuzmine & Craig T. Martin, J. Mol. Biol. 305, 559-566, 2001
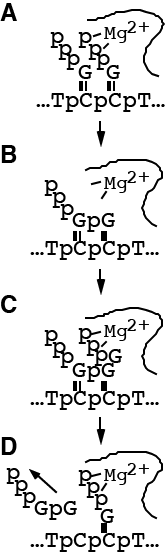
In order to begin to understand the mechanism of the initiation of transcription in the model T7 RNA polymerase system, the simplest possible reaction, the synthesis of dinucleotide, has been followed by quench-flow kinetics, and numerical integration of mechanism-specific rate equations has been used to test specific kinetic models. In order to fit the observed time dependence in the pre-steady state kinetics, a model for dinucleotide synthesis is proposed in which rebinding of the dinucleotide to the enzyme-DNA complex must be included. Separate reactions using dinucleotide as a substrate confirm this mechanism and the determined rate constants. The dinucleotide rebinding observed as inhibition under these conditions forms a productive intermediate in the synthesis of longer transcripts, and must be included in future kinetic mechanisms. The rate limiting step leading to product formation shows a substrate dependence consistent with the binding of two substrate GTP molecules, and at saturating levels of GTP, is comparable in magnitude to the product release rate. The rate of product release shows a positive correlation with the concentration of GTP, suggesting that the reaction shows base-specific substrate activation. The binding of another substrate molecule, presumably via interaction with the triphosphate binding site, likely facilitates displacement of the dinucleotide product from the complex. A printer error at the journal omitted two equations in the PDF version of the manuscript. Download page with the correct equations here.
PMID: 11152612 DOI: 10.1006/jmbi.2000.4316